Latest Version
Version
5.0.mRfx
5.0.mRfx
Update
October 23, 2024
October 23, 2024
Developer
Logiciel Puissant
Logiciel Puissant
Categories
Education
Education
Platforms
Android
Android
Downloads
0
0
License
Free
Free
Package Name
compromdompango.llsoft.chemistryolevelnotes
compromdompango.llsoft.chemistryolevelnotes
Report
Report a Problem
Report a Problem
More About Chemistry O Level Notes
Included topics:
Soil:
This topic covers the study of soil, including its composition, properties, and importance in agriculture and the environment.
Introduction to Organic Chemistry:
Organic Chemistry is the study of carbon-containing compounds. Students learn about the properties, nomenclature, and reactions of organic compounds.
Non-Metals and Their Compounds - General Chemical Properties of Non-Metals:
This topic explores the general chemical properties of non-metals, including their reactivity, reaction with oxygen, hydrogen, and water, and their acidic nature.
Compounds of Metals:
This topic covers the properties, preparation, and uses of compounds of metals, including oxides, hydroxides, and salts.
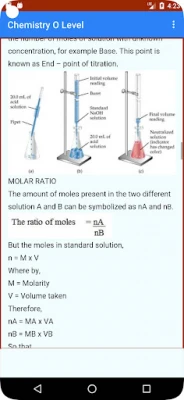
Quantitative Analysis and Volumetric Analysis:
Quantitative Analysis involves the determination of the quantity or concentration of substances in a sample. Volumetric Analysis focuses on measuring volumes in chemical reactions, often involving titrations.
Chemical Kinetics, Equilibrium, and Energetics - Rate of Reaction:
Chemical Kinetics is the study of reaction rates and factors affecting reaction rates, including the rate equation and rate-determining steps.
Chemical Kinetics, Equilibrium, and Energetics - Equilibrium and Energetics:
This subtopic delves into chemical equilibrium, Le Chatelier's principle, and the relationship between energy changes and chemical reactions.
Hardness of Water:
Hardness of Water deals with the presence of calcium and magnesium ions in water and its impact on soap usage and industrial processes.
Ionic Theory and Electrolysis - Electrolysis:
Ionic Theory involves the concept of ions in chemical reactions. Electrolysis is the process of using electricity to drive a non-spontaneous chemical reaction.
Mole Concept:
The Mole Concept is a fundamental concept in chemistry that relates the amount of substance to its mass and the Avogadro constant.
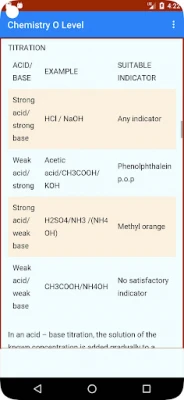
Acids, Bases, and Salt - Chemical Equation:
This topic covers the reactions of acids and bases, their properties, and the formation of salts.
Fuel:
Fuel focuses on various types of fuels, their combustion, and energy production.
Periodic Classification - Atomic Structure:
The Periodic Classification topic relates to the organization of elements in the periodic table and the atomic structure of elements.
Water, Hydrogen, Oxygen, and Air:
These subtopics cover the properties, uses, and importance of water, hydrogen, oxygen, and air in various chemical processes.
Combustion, Rusting, and Firefighting:
This topic explores combustion reactions, rusting of metals, and the principles of firefighting.
Laboratory Technique and Safety:
Laboratory Technique and Safety are essential components of practical chemistry, emphasizing proper laboratory practices and safety precautions.
Matter:
Matter involves the study of different states of matter and their properties.
Heat Sources and Flames:
This topic covers the sources of heat and types of flames produced in various combustion reactions.
Scientific Procedure - Introduction to Chemistry:
This topic introduces students to the scientific method and its application in the field of chemistry.
This topic covers the study of soil, including its composition, properties, and importance in agriculture and the environment.
Introduction to Organic Chemistry:
Organic Chemistry is the study of carbon-containing compounds. Students learn about the properties, nomenclature, and reactions of organic compounds.
Non-Metals and Their Compounds - General Chemical Properties of Non-Metals:
This topic explores the general chemical properties of non-metals, including their reactivity, reaction with oxygen, hydrogen, and water, and their acidic nature.
Compounds of Metals:
This topic covers the properties, preparation, and uses of compounds of metals, including oxides, hydroxides, and salts.
Quantitative Analysis and Volumetric Analysis:
Quantitative Analysis involves the determination of the quantity or concentration of substances in a sample. Volumetric Analysis focuses on measuring volumes in chemical reactions, often involving titrations.
Chemical Kinetics, Equilibrium, and Energetics - Rate of Reaction:
Chemical Kinetics is the study of reaction rates and factors affecting reaction rates, including the rate equation and rate-determining steps.
Chemical Kinetics, Equilibrium, and Energetics - Equilibrium and Energetics:
This subtopic delves into chemical equilibrium, Le Chatelier's principle, and the relationship between energy changes and chemical reactions.
Hardness of Water:
Hardness of Water deals with the presence of calcium and magnesium ions in water and its impact on soap usage and industrial processes.
Ionic Theory and Electrolysis - Electrolysis:
Ionic Theory involves the concept of ions in chemical reactions. Electrolysis is the process of using electricity to drive a non-spontaneous chemical reaction.
Mole Concept:
The Mole Concept is a fundamental concept in chemistry that relates the amount of substance to its mass and the Avogadro constant.
Acids, Bases, and Salt - Chemical Equation:
This topic covers the reactions of acids and bases, their properties, and the formation of salts.
Fuel:
Fuel focuses on various types of fuels, their combustion, and energy production.
Periodic Classification - Atomic Structure:
The Periodic Classification topic relates to the organization of elements in the periodic table and the atomic structure of elements.
Water, Hydrogen, Oxygen, and Air:
These subtopics cover the properties, uses, and importance of water, hydrogen, oxygen, and air in various chemical processes.
Combustion, Rusting, and Firefighting:
This topic explores combustion reactions, rusting of metals, and the principles of firefighting.
Laboratory Technique and Safety:
Laboratory Technique and Safety are essential components of practical chemistry, emphasizing proper laboratory practices and safety precautions.
Matter:
Matter involves the study of different states of matter and their properties.
Heat Sources and Flames:
This topic covers the sources of heat and types of flames produced in various combustion reactions.
Scientific Procedure - Introduction to Chemistry:
This topic introduces students to the scientific method and its application in the field of chemistry.
Rate the App
Add Comment & Review
User Reviews
Based on 0 reviews
No reviews added yet.
Comments will not be approved to be posted if they are SPAM, abusive, off-topic, use profanity, contain a personal attack, or promote hate of any kind.
More »










Popular Apps

Santander Inversiones Uruguay 5Banco Santander Uruguay

Santander Empresas Portugal 5Banco Santander Totta S.A.

Mi Tarjeta SantanderBanco Santander Uruguay

Santander InternationalSantander International

Santander ArgentinaBanco Santander (Argentina)
Santander EmpresasBanco Santander (Brasil) S.A.

RollerCoaster Tycoon® Classic 5Atari, Inc.

SantanderSignSantander Consumer Bank AG (Deutschland)

Santander mobileSantander Bank Polska S.A.

Santander Way: App de cartõesBanco Santander (Brasil) S.A.
More »










Editor's Choice

Grim Soul: Dark Survival RPG 5Brickworks Games Ltd

Craft of Survival - Gladiators 5101XP LIMITED

Last Shelter: Survival 5Long Tech Network Limited

Dawn of Zombies: Survival GameRoyal Ark

Merge Survival : Wasteland 5StickyHands Inc.

AoD Vikings: Valhalla Game 5RoboBot Studio

Viking Clan: Ragnarok 5Kano Games

Vikings: War of Clans 5Plarium LLC

Asphalt 9: Legends 5Gameloft SE

Modern Tanks: War Tank Games 5XDEVS LTD